Nivolumab + chemo – a candidate for a new standard 1L treatment of advanced gastric/esophageal adenocarcinoma
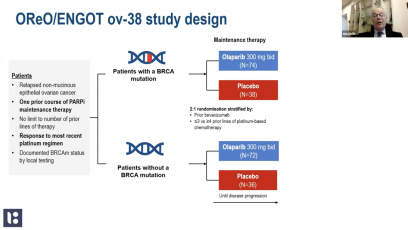
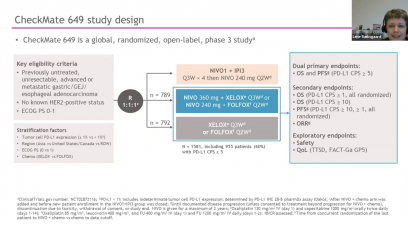
At a Presidential symposium during ESMO 2021, Yelena Janjigian presented the data from CheckMate 649 study – a randomized, global phase 3 study of 1L programmed death (PD) 1 inhibitor-based therapies in advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma. The results of CheckMate 649 was lading to US FDA approval. Based on the results of CheckMate 649, the treatment received FDA approval. In this MEDtalk Yelena Janjigian explains, why these data further support nivolumab in combination with chemo therapy, as a new standard 1L treatment .