Prospects for the Use of Antibodies Against Parkinson’s Disease: A Multi-Edged Sword?
Antibodies are amazing inventions of Nature, designed by the immune system to rapidly and specifically target virtually any foreign or harmful entity encountered by the body. Their structure is tailored to balance recognition and activation for optimal defense, providing the immune system with both specificity and broad-range reactivity.
This is maintained by two important features: (1) molecular recognition in the variable domains (which by themselves can suppress undesirable events by binding to toxic target molecules) and (2) a constant domain that can activate cellular defenses by marshalling macrophages, neutrophils, and natural killer cells to engulf and degrade the target molecules/pathogens, or can facilitate attack of the complement system.
The Blood-Brain Barrier is a Challenge for Monoclonal Antibodies
Unfortunately, while monoclonal antibodies (mAbs) have achieved spectacular success against non-neurological diseases such as cancers and inflammatory disorders, they have been much less successful against diseases of the central nervous system (CSN). This is particularly the case for conformational diseases such as Alzheimer’s (AD) and Parkinson’s (PD). They both involve aggregation of proteins, the amyloid-β (Aβ) peptide in the case of AD and the intrinsically disordered protein α-synuclein (α-syn) in PD. These proteins accumulate inside or on the surface of neurons in the form of insoluble fibrils and soluble oligomers, which can spread to neighboring neurons, driving further aggregation1. Many small molecules and antibodies targeting and blocking this aggregation have been developed and tested, yet without clear clinical promise2. Indeed, three clinical trials for PD driven by mAbs directed against α-syn were recently terminated without a successful outcome3. While the specific reasons for the inability to meet milestones were not clear, one of the most significant hurdles to overcome in these trials is the blood-brain barrier (BBB).
Three Necessary Advancements
The BBB is a functional and structural barrier at cerebral microvessels that protects the brain from most blood-borne substances. Diffusion across the BBB is possible but restricted to low-molecular-weight hydrophilic and hydrophobic compounds and countered by rapid outward transport by efflux pumps4. Proteins may enter by vesicular transport (transcytosis), but this route is highly selective and actively suppressed5. Consequently, the BBB precludes >99% of neuroprotective compounds from reaching the brain, rendering CNS disorders resistant to conventional therapeutic approaches6. Furthermore, many parts of our defenses, such as antibodies, neutrophils, T cells, and the complement system, are excluded from the brain parenchyma, making it difficult to mobilize these otherwise powerful molecular tools to eliminate protein aggregates.
To fully leverage the potential of therapeutic mAbs, three critical advancements are needed. Firstly, we must improve methods to deliver antibodies across the BBB. Secondly, the mAbs must be optimized not just to bind to their targets but also to actively promote target elimination. Thirdly, we need reliable methods to diagnose the extent and distribution of α-syn aggregation in live patients. Let us consider each in turn.
Aggregation of α-syn to toxic oligomers and fibrils is one of the central events in the development of PD and other synucleinopathies, presenting an attractive therapeutic target.
Brain Delivery Systems
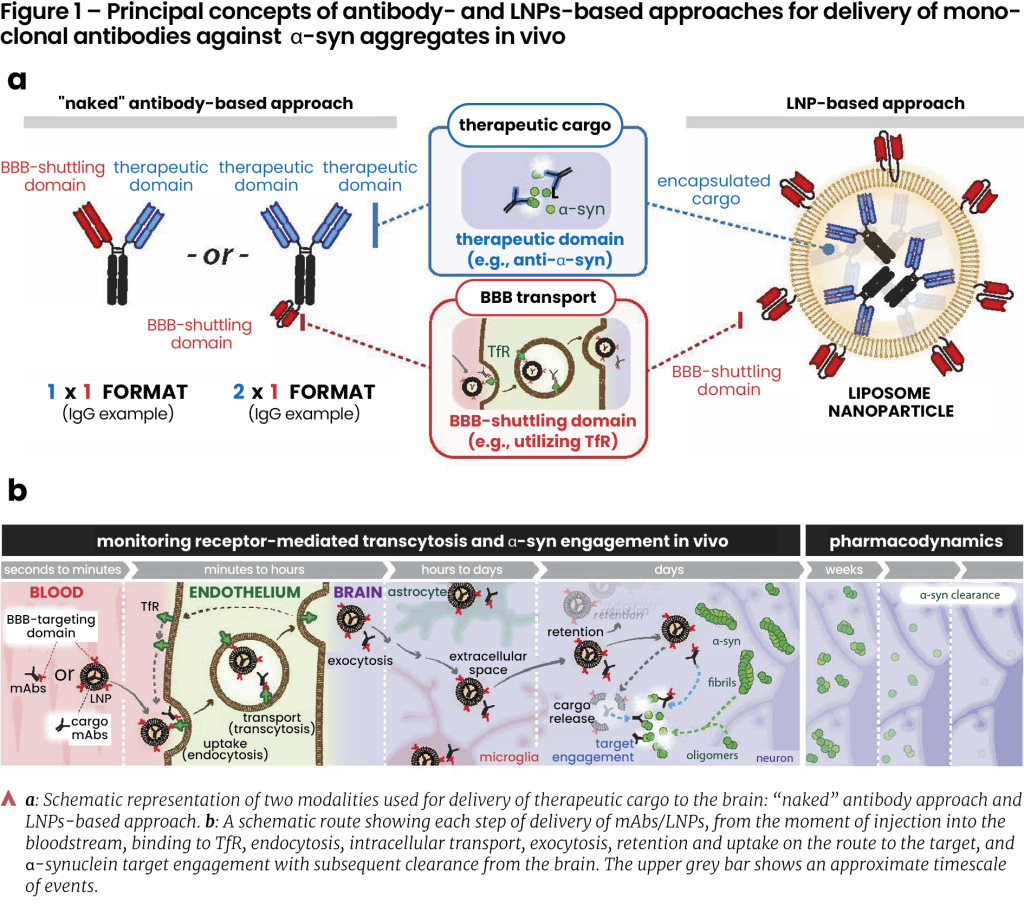
Most current approaches for targeted drug delivery aim to utilize receptor-mediated transcytosis, a type of vesicular transport in endothelium. A popular approach to move cargo across the BBB is to use the transferrin receptor (TfR), a densely expressed ferrying receptor on brain endothelial cells (BECs)7-9. For instance, mAbs cross the BBB to a greater extent if they can “hijack” the TfR-mediated transport. This is achieved by “stapling” mAbs with ligands that bind to the TfR. To avoid blocking the TfR’s interactions with its natural substrate, transferrin, these ligands are often fragments of other antibodies that recognize the TfR10-12.
Another paradigm, in which we expect to increase the delivery levels considerably, is to package the mAbs inside functionalized lipid nanoparticles (LNPs). LNPs are liposomes supplemented with ionizable lipids, cholesterol, and PEG groups that enhance their stability, circulation time, and reduce unwanted interactions with the immune system13, 14. Importantly, coupling TfRs ligands to the LNPs allows utilization of transcytosis, which leads to greatly enhanced brain uptake, making them versatile delivery vehicles able to encapsulate large payloads of mAbs. Such LNPs have already shown promise in preclinical trials in brain cancer15, and stroke16, as well as in PD and other CNS diseases17; achieving ~2 orders of magnitude higher delivery to the brain compared to unfunctionalized (non-targeted) LNPs16.
The LNP strategy has also recently been shown to outperform “naked” TfR-targeting mAbs in crossing the BBB and delivering anti-α-syn mAbs that inhibit α-syn aggregation13. The two approaches are summarized in Figure 1. Despite these advancements, further optimization of LNPs and trans-BBB delivery routes is needed to achieve delivery levels of clinical relevance.
The group of Nikos Hatzakis has pioneered fluorescence-based microscopy methods to screen and optimize the loading of LNPs with cargo and to follow their delivery18, 19, building a platform to systematically analyse LNPs with machine learning techniques18, 20. This allows bulk analyses of LNP trafficking over brain endothelial cells and uniquely quantifies how the LNPs distribute themselves on the membrane, internalize through different cellular pathways, and release their cargo20. Such insights are essential because although LNPs have demonstrated the ability to cross the BBB21, 22, we have limited or no understanding of the mechanism. This imposes a challenge for the design of efficient drug delivery strategies. Following this optimization, the therapeutic potential of these LNPs can then be tested in cells from PD patients that display α-syn pathology as developed by the group of Céline Galvagnion23. Once the right LNPs have been identified, it is possible to follow their transport over the BBB into the brain parenchyma using 2-photon microscopy on live awake mice, as demonstrated by the group of Martin Lauritzen and Krzysztof Kucharz24, 25. We hope that all this will allow us to identify LNPs that can boost delivery levels to make a therapeutic impact within our research consortium NanoPANS (Functionalized Nanocarriers sending anti-PArkinson’s Disease drugs to the Nervous System), recently funded by the Lundbeck Foundation.
Engineered Aggregate-Binding Antibodies
Aggregation of α-syn to toxic oligomers and fibrils is one of the central events in the development of PD and other synucleinopathies, presenting an attractive therapeutic target. Therapy is, however, complicated by the fact that different subtypes of these diseases can have different distributions and types of aggregates. It is, therefore, important to have access to a range of antibodies that are able to recognize all specific types of these aggregates and eliminate them. Yet, this is no simple matter. Fibrils and oligomers of α-syn consist of dynamic and disordered regions along with domains of atomically well-defined and persistent structures, arranged in a repetitive pattern. The C-terminal part of α-syn, which is highly immunogenic and a typical binding site for mAbs, is disordered both in the monomeric, oligomeric, and fibrillar state, so a mAb targeting this region will be able to bind all aggregated states and will only prefer fibrils and oligomers due to avidity, i.e., a high local concentration of binding sites. It is therefore important to carry out a thorough analysis of the mAbs’ binding preferences to build up a molecular understanding of how they recognize α-syn aggregation. Unlike immobilization techniques such as ELISA, which are prone to artifacts, in-solution methods provide more reliable insights. Using such in-solution techniques, Daniel Otzen’s group has recently reported the biophysical and immunohistochemical profiles of 30 different mAbs2 which we raised in mice using different forms of oligomeric α-syn. These mAbs target the whole range of α-syn aggregates expected in PD in vivo with a broad range of affinities and specificities for fibrillated, oligomeric, and monomeric α-syn. Several of these mAbs could identify α-syn pathologies in postmortem PD brains, including early stages of the disease. Notably, the great variation in the binding profile of these mAbs illustrated the need for a careful and comparative evaluation of binding properties and histological profiles of the different mAbs currently under consideration for clinical trials, as argued both by Lashuel et al.26 and our own research2, 27.
Development of Antibodies into More Effective Treatment
By immunizing llamas with our α-syn oligomers, we have also raised single-domain antibodies or nanobodies against these aggregates28. Unlike our mAbs, these nanobodies show absolute specificity for the oligomers and do not bind to monomers or fibrils. It is useful to be able to distinguish between oligomers and fibrils since their relative roles in the development of PD are still a subject of considerable debate1 and it has traditionally been difficult to detect oligomeric species in vivo. In contrast, fibrils can be quantified with impressive accuracy using a seed amplification assay that is based on patient cerebrospinal fluid29.
Once we have access to antibodies against the α-syn aggregates, it would be helpful to engineer them not only to bind but also to effectively eliminate their targets so that all toxicity is eliminated. A very promising approach would be to fuse various degradation-signaling peptides to the antibodies in such a way that they do not interfere with target engagement but direct the antibody-target complex to a suitable degradation pathway, e.g., the proteasome (PROTAC) or the lysosome (LYTAC), where “TAC” stands for “Targeting Chimeras”30. Since both oligomers and fibrils of α-syn are too large for the proteasome to process, the PROTAC strategy is not likely to work. However, larger organelles such as lysosomes and autosomes are more promising and can be targeted using, e.g., short motifs binding to the chaperone Hsc7031, the receptor binding domain from apolipoprotein B32, or the sortilin receptor33. These innovations could transform antibodies into more effective therapeutic tools.
Diagnosing PD and Other α-Synucleinopathies at Earlier Stages
Ideally, PD and other α-synucleinopathies should be detected early enough to allow therapeutic intervention even prior to the development of clinical symptoms. This is not yet the case. The seed amplification assay holds promise but uses cerebrospinal fluid and does not probe the brain itself. Detection of α-syn aggregates in the brain would be possible if mAbs binding these aggregates could cross into the brain tissue and be monitored using PET ligands. This is currently not possible because there is no α-syn-specific PET ligand available. Here, α-syn-specific mAbs are an obvious tool to employ. It would be hugely encouraging to develop new PET tracers that are sufficiently long-lived to allow the PET-labelled antibodies to reach steady-state distribution in the body and to be evaluated using total-body PET for distribution within and outside the brain. This PET-mAb approach could also be used to examine and distinguish the two major PD subtypes proposed by Van den Berge and Borghammer34: In body-first PD, α-syn pathology originates in the gut’s enteric nervous system and invades the brain through the sympathetic and parasympathetic system. In contrast, brain-first PD pathology starts in the olfactory bulb and limbic system, later spreading to the brainstem and PNS. Obviously, this approach could also be extended to other CNS diseases if combined with the appropriate mAbs.
We expect that the next few years will yield progress along all three fronts, i.e. developing better delivery systems using functionalized (receptor-targeting) nanolipid particles, optimizing antibodies to target both α-syn aggregates and the sortilin receptor for intracellular degradation and developing new PET isotopes to label these antibodies for diagnostics. All in all, there is reason to be both hopeful and excited about the prospects for using antibodies to combat PD and other neurodegenerative diseases, not least when combined with the many other efforts made to combat these devastating diseases.
-
Acknowledgments
We are grateful to Nathalie van den Berge, Dirk Bender, and Per Borghammer (Director of the new Lundbeck Foundation Parkinson’s Disease Research Center PACE) for ongoing discussions on PET tracers and PD subtypes.
-
Conflicts of interest
None.
-
References
- P. Alam, L. Bousset, R. Melki, D.E. Otzen, α-synuclein oligomers and fibrils: a spectrum of species, a spectrum of toxicities, J Neurochem 150(5) (2019) 522-534.
- J. Nielsen, J. Lauritsen, J.N. Pedersen, J.S. Nowak, M.K. Bendtsen, G. Kleijwegt, K. Lusser, L.C. Pitarch, J.V. Moreno, M.M. Schneider, G. Krainer, L. Goksøyr, P. Khalifé, S.S. Kaalund, S. Aznar, K. Kjærgaard, V. Sereikaité, K. Strømgaard, T.P.J. Knowles, M. Agertoug Nielsen, A.F. Sander, M. Romero-Ramos, D.E. Otzen, Monoclonal antibodies targeting cytotoxic α-synuclein oligomers: molecular properties and diagnostic potential, NPJ Parkinson's Disease 10(1) (2024) 139.
- E.M. Prasad, S.Y. Hung, Current Therapies in Clinical Trials of Parkinson's Disease: A 2021 Update, Pharmaceuticals (Basel) 14(8) (2021).
- W.M. Pardridge, Drug Transport across the Blood–Brain Barrier, Journal of Cerebral Blood Flow & Metabolism 32(11) (2012) 1959-1972.
- A. Ben-Zvi, B. Lacoste, E. Kur, B.J. Andreone, Y. Mayshar, H. Yan, C. Gu, Mfsd2a is critical for the formation and function of the blood–brain barrier, Nature 509(7501) (2014) 507-511.
- W.M. Pardridge, Drug and Gene Delivery to the Brain: The Vascular Route, Neuron 36(4) (2002) 555-558.
- T.M. Allen, P.R. Cullis, Drug Delivery Systems: Entering the Mainstream, Science 303(5665) (2004) 1818-1822.
- W.M. Pardridge, Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain, Frontiers in Aging Neuroscience 11 (2020).
- K.B. Johnsen, A. Burkhart, L.B. Thomsen, T.L. Andresen, T. Moos, Targeting the transferrin receptor for brain drug delivery, Progress in Neurobiology 181 (2019) 101665.
- G. Hultqvist, S. Syvänen, X.T. Fang, L. Lannfelt, D. Sehlin, Bivalent Brain Shuttle Increases Antibody Uptake by Monovalent Binding to the Transferrin Receptor, Theranostics 7(2) (2017) 308-318.
- M.S. Kariolis, R.C. Wells, J.A. Getz, W. Kwan, C.S. Mahon, R. Tong, D.J. Kim, A. Srivastava, C. Bedard, K.R. Henne, T. Giese, V.A. Assimon, X. Chen, Y. Zhang, H. Solanoy, K. Jenkins, P.E. Sanchez, L. Kane, T. Miyamoto, K.S. Chew, M.E. Pizzo, N. Liang, M.E.K. Calvert, S.L. DeVos, S. Baskaran, S. Hall, Z.K. Sweeney, R.G. Thorne, R.J. Watts, M.S. Dennis, A.P. Silverman, Y.J.Y. Zuchero, Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys, Science Translational Medicine 12(545) (2020) eaay1359.
- J.C. Ullman, A. Arguello, J.A. Getz, A. Bhalla, C.S. Mahon, J. Wang, T. Giese, C. Bedard, D.J. Kim, J.R. Blumenfeld, N. Liang, R. Ravi, A.A. Nugent, S.S. Davis, C. Ha, J. Duque, H.L. Tran, R.C. Wells, S. Lianoglou, V.M. Daryani, W. Kwan, H. Solanoy, H. Nguyen, T. Earr, J.C. Dugas, M.D. Tuck, J.L. Harvey, M.L. Reyzer, R.M. Caprioli, S. Hall, S. Poda, P.E. Sanchez, M.S. Dennis, K. Gunasekaran, A. Srivastava, T. Sandmann, K.R. Henne, R.G. Thorne, G. Di Paolo, G. Astarita, D. Diaz, A.P. Silverman, R.J. Watts, Z.K. Sweeney, M.S. Kariolis, A.G. Henry, Brain delivery and activity of a lysosomal enzyme using a blood-brain barrier transport vehicle in mice, Science Translational Medicine 12(545) (2020) eaay1163.
- M. Sela, M. Poley, P. Mora-Raimundo, S. Kagan, A. Avital, M. Kaduri, G. Chen, O. Adir, A. Rozencweig, Y. Weiss, O. Sade, Y. Leichtmann-Bardoogo, L. Simchi, S. Aga-Mizrachi, B. Bell, Y. Yeretz-Peretz, A.Z. Or, A. Choudhary, I. Rosh, D. Cordeiro, S. Cohen-Adiv, Y. Berdichevsky, A. Odeh, J. Shklover, J. Shainsky-Roitman, J.E. Schroeder, D. Hershkovitz, P. Hasson, A. Ashkenazi, S. Stern, T. Laviv, A. Ben-Zvi, A. Avital, U. Ashery, B.M. Maoz, A. Schroeder, Brain-Targeted Liposomes Loaded with Monoclonal Antibodies Reduce Alpha-Synuclein Aggregation and Improve Behavioral Symptoms in Parkinson's Disease, Advanced Materials 35(51) (2023) 2304654.
- R. Tenchov, R. Bird, A.E. Curtze, Q. Zhou, Lipid Nanoparticles - From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement, ACS Nano 15(11) (2021) 16982-17015.
- J.P. Straehla, C. Hajal, H.C. Safford, G.S. Offeddu, N. Boehnke, T.G. Dacoba, J. Wyckoff, R.D. Kamm, P.T. Hammond, A predictive microfluidic model of human glioblastoma to assess trafficking of blood–brain barrier-penetrant nanoparticles, Proceedings of the National Academy of Sciences 119(23) (2022) e2118697119.
- J. Nong, P.M. Glassman, V.V. Shuvaev, S. Reyes-Esteves, H.C. Descamps, R.Y. Kiseleva, T.E. Papp, M.-G. Alameh, Y.K. Tam, B.L. Mui, S. Omo-Lamai, M.E. Zamora, T. Shuvaeva, E. Arguiri, X. Gong, T.V. Brysgel, A.W. Tan, A.G. Woolfork, A. Weljie, C.A. Thaiss, J.W. Myerson, D. Weissman, S.E. Kasner, H. Parhiz, V.R. Muzykantov, J.S. Brenner, O.A. Marcos-Contreras, Targeting lipid nanoparticles to the blood-brain barrier to ameliorate acute ischemic stroke, Molecular Therapy 32(5) (2024) 1344-1358.
- P. Khare, S.X. Edgecomb, C.M. Hamadani, E.E.L. Tanner, D. S Manickam, Lipid nanoparticle-mediated drug delivery to the brain, Advanced Drug Delivery Reviews 197 (2023) 114861.
- H.D. Pinholt, S.S. Bohr, J.F. Iversen, W. Boomsma, N.S. Hatzakis, Single-particle diffusional fingerprinting: A machine-learning framework for quantitative analysis of heterogeneous diffusion, Proc Natl Acad Sci U S A 118(31) (2021).
- G. Huang, M.W. Dreisler, J. Kæstel-Hansen, A.J. Nielsen, M. Zhang, N.S. Hatzakis, Defect-Engineered Metal–Organic Frameworks as Nanocarriers for Pharmacotherapy: Insights into Intracellular Dynamics at The Single Particle Level, Advanced Materials 36(35) (2024) 2405898.
- J. Kæstel-Hansen, M. de Sautu, A. Saminathan, G. Scanavachi, R.F.B. Da Cunha Correia, A.J. Nielsen, S.V. Bleshøy, W. Boomsma, T. Kirchhausen, N.S. Hatzakis, Deep learning assisted single particle tracking for automated correlation between diffusion and function, bioRxiv (2023) 2023.11.16.567393.
- I. Arduino, N. Depalo, F. Re, R. Dal Magro, A. Panniello, N. Margiotta, E. Fanizza, A. Lopalco, V. Laquintana, A. Cutrignelli, A.A. Lopedota, M. Franco, N. Denora, PEGylated solid lipid nanoparticles for brain delivery of lipophilic kiteplatin Pt(IV) prodrugs: An in vitro study, International Journal of Pharmaceutics 583 (2020) 119351.
- M. Qu, Q. Lin, S. He, L. Wang, Y. Fu, Z. Zhang, L. Zhang, A brain targeting functionalized liposomes of the dopamine derivative N-3,4-bis(pivaloyloxy)-dopamine for treatment of Parkinson's disease, Journal of Controlled Release 277 (2018) 173-182.
- S.S. Muñoz, D. Petersen, F.R. Marlet, E. Kücükköse, C. Galvagnion, The interplay between Glucocerebrosidase, α-synuclein and lipids in human models of Parkinson’s disease, Biophysical Chemistry 273 (2021) 106534.
- K. Kucharz, K. Kristensen, K.B. Johnsen, M.A. Lund, M. Lønstrup, T. Moos, T.L. Andresen, M.J. Lauritzen, Post-capillary venules are the key locus for transcytosis-mediated brain delivery of therapeutic nanoparticles, Nature Comm. 12 (2021) 4121.
- K. Kucharz, N. Kutuzov, O. Zhukov, M. Mathiesen Janiurek, M. Lauritzen, Shedding Light on the Blood-Brain Barrier Transport with Two-Photon Microscopy In Vivo, Pharm Res 39(7) (2022) 1457-1468.
- S.T. Kumar, S. Jagannath, C. Francois, H. Vanderstichele, E. Stoops, H.A. Lashuel, How specific are the conformation-specific α-synuclein antibodies? Characterization and validation of 16 α-synuclein conformation-specific antibodies using well-characterized preparations of α-synuclein monomers, fibrils and oligomers with distinct structures and morphology, Neurobiol Dis 146 (2020) 105086.
- D.E. Otzen, Antibodies and α-synuclein: What to target against Parkinson’s Disease?, Biochim Biophys Acta Proteins Proteomics 1872 (2023) 140943.
- J. Nielsen, J.N. Pedersen, G. Kleijwegt, J.S. Nowak, F. Nami, C. Johansen, E. Sassetti, B.B. Bjerre, N.M. Lyngsø, B.V. Brøchner, J.H. Carlson, A. Simonsen, W.P. Olsen, B.W. Simonsen, J.H. Mikkelsen, V. Sereikaité, M.G. Malle, A. Bøggild, T. Boesen, S. Birkelund, G. Christiansen, S.B. Hansen, P.S. Madsen, K. Strømgaard, C. Gustafsen, S. Glerup, K.R. Andersen, M.H. Clausen, D.E. Otzen, Nanobodies raised against the cytotoxic α-synuclein oligomer are specific for the oligomeric species and promote its cellular uptake, npj biosensing Submitted (2024).
- A. Siderowf, L. Concha-Marambio, K. Marek, C. Soto, α-synuclein seed amplification in Parkinson's disease - Authors' reply, The Lancet Neurology 22(11) (2023) 985-986.
- L. Zhao, J. Zhao, K. Zhong, A. Tong, D. Jia, Targeted protein degradation: mechanisms, strategies and application, Signal Transduction and Targeted Therapy 7(1) (2022) 113.
- A.M. Cuervo, E. Wong, Chaperone-mediated autophagy: roles in disease and aging, Cell Research 24(1) (2014) 92-104.
- B. Spencer, S. Emadi, P. Desplats, S. Eleuteri, S. Michael, K. Kosberg, J. Shen, E. Rockenstein, C. Patrick, A. Adame, T. Gonzalez, M. Sierks, E. Masliah, ESCRT-mediated uptake and degradation of brain-targeted α-synuclein single chain antibody attenuates neuronal degeneration in vivo, Mol Ther 22(10) (2014) 1753-67.
- F. Hu, T. Padukkavidana, C.B. Vægter, O.A. Brady, Y. Zheng, I.R. Mackenzie, H.H. Feldman, A. Nykjaer, S.M. Strittmatter, Sortilin-Mediated Endocytosis Determines Levels of the Frontotemporal Dementia Protein, Progranulin, Neuron 68(4) (2010) 654-667.
- P. Borghammer, N. Van Den Berge, Brain-First versus Gut-First Parkinson's Disease: A Hypothesis, J Parkinsons Dis 9(s2) (2019) S281-S295.
Brænder du for at skrive?
Vil du gerne dele din forskning eller dine kliniske erfaringer med dine kollegaer inden for netop dit speciale? Har du en ide til en artikel, som du gerne vil udgive hos os? Send redaktionen en mail på redaktion@bpno.dk
Send mail til redaktionen









